Benefits of autogenous vaccines
The pluses of Ceva Biovac:
Prevent rather than cure
- Limit disease occurrence
- Deal with multi-resistant bacteria
This is the function of every vaccine; the development of bacterial resistance has abolished the option of antibiotic treatment.
Contribute to public health:
- Reduce excretion of the microbial toxins that cause infection
- Limit the use of anti-infective agents
The food-processing industry has a duty to protect consumer health.
Provide solutions where there are no vaccines:
- Vaccinate as a matter of urgency in the case of emerging diseases
- Propose solutions for diseases described as “minor” and for species designated as “secondary”
The time and cost involved in developing a commercial vaccine are sometimes incompatible with the demands of those working on the ground and of the target markets.
Obtain specific immunity:
- Fight against vaccination failure
Antigenic variability within the same bacterial species, within the same serotype even, is such that total protection is often unpredictable when the vaccine antigen differs from the aggressor.
Reduce the costs of production:
- Limit the number of vaccine interventions by combining several valencies
- Develop innovative pharmaceutical forms that reduce human interventions
The cost of handling the animals and the resulting inconveniences for those involved as well as the stress caused to the animals themselves demand a commitment to simplify plans for prophylaxis: the number of injections can be reduced by combining several valencies and excellent protection can be obtained in some cases with a single administration by developing high-performance adjuvants. Autogenous vaccines adapted to oral administration can be used in certain indications.
Preparation
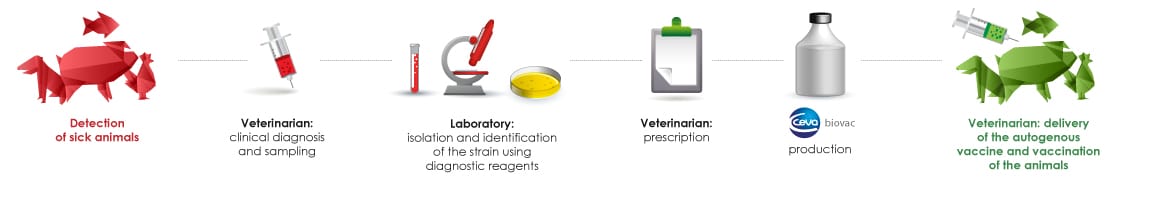
Process for developing autogenous vaccines
The veterinarian takes a sample, either from a sick animal or during an autopsy, which he sends to a diagnostic laboratory for culture. In the laboratory, the pathogenic agent is isolated, identified and serotyped using reagents produced by Ceva Biovac. The veterinarian then orders the manufacture of an autogenous vaccine from the isolated strain which is then sent to Ceva Biovac. The autogenous vaccine produced by Ceva Biovac in 4 to 6 weeks depending on its composition, is supplied to the veterinarian who issues it to his client. Ceva Biovac is also available to help veterinarians develop a vaccination programme.
Through its products and services, Ceva Biovac offers a complete and custom-made solution to the livestock sector and the veterinary profession.
Produced in state-of-the-art biotechnology facilities according to draconian standards of sterility, the Ceva Biovac autogenous vaccines can be used in multiple indications and are intended for all animal species (apart from ruminants), ranging from livestock to pets.
Regulation
BACTERIAL AUTOGENOUS VACCINES
Article L 5141-2 of the Public Health Code:
“Autogenous vaccines for veterinary use are defined as any immunological veterinary medicinal products manufactured for the purpose of producing active immunity from pathogenic organisms obtained from an animal or animals from the same herd that have been inactivated and used for the treatment of this animal or of animals from this herd”.
Article L 5141-12 of the Public Health Code:
“The preparation of autogenous vaccines for veterinary use should be undertaken by a qualified person or company or organisation employing a qualified person who has obtained for this purpose a licence issued by the French Food Safety Agency”.
Decree no. 2005-374 of 20 April 2005 about autogenous vaccines for veterinary use in amendment of the Public Health Code (regulatory part)
Art. 5141-129. − The preparation of autogenous vaccines is undertaken under the responsibility of a qualified person who has obtained the licence provided for in article L. 5141-12. (...)
“The qualified person is a pharmacist or veterinarian. (...) “The qualified person can show that he or she has undergone professional training or has professional experience in the domain of immunology or the manufacture of medicinal products. He or she exercises his or her functions personally. He or she monitors the activities involved in the preparation, storage, transport and monitoring of autogenous vaccines for veterinary use in accordance with good practice as defined by order of the ministers responsible for agriculture and health issued on the proposal of the general director of the French Food Safety Agency”.
“Art. R. 5141-141. − The holder of the permit provided for in article L. 5141-12 can supply an autogenous vaccine for veterinary use only to a prescribing veterinarian or to any other veterinarian who has given, on inclusion in the order, the same business address as defined in article R. 242-52 of Rural Code.”
Order of 6 March 2008 about Good Preparation Practice for autogenous vaccines for veterinary use:
Gives details of Good Preparation Practice (GPP) for autogenous vaccines for veterinary use referred to in article no. 5141-129 of the above decree. The permit provided for in article L.5141-12 is granted to Ceva BIOVAC 6 rue Olivier de Serres, 49070 Beaucouzé, for the preparation of autogenous vaccines for veterinary use at the premises situated at the same address. Registered under no. AV 0901/07, the permit specifies the authorised pathogenic agents by target species and the authorised pharmaceutical forms and adjuvants.
Display the GPP certificate

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam